The VSD project includes a large linked database that uses administrative data sources at each MCO. Each participating site gathers data on vaccination (vaccine type, date of vaccination, concurrent vaccinations), medical outcomes (outpatient visits, inpatient visits, urgent care visits), birth data, and census data.
The VSD project allows for planned immunization safety studies as well as timely investigations of hypotheses that arise from review of medical literature, reports to the Vaccine Adverse Event Reporting System (VAERS), changes in immunization schedules, or the introduction of new vaccines.
Since 1990, investigators from the VSD project have published more than 75 scientific articles. The VSD project has addressed the following immunization safety concerns:
- A study that quantifies the risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella (MMR) vaccine
- An investigation of MMR and other measles containing vaccines which found no increased risk for inflammatory bowel disease
- An examination of the safety of a vaccination with the pneumococcal polysaccharide vaccine
- An evaluation of the VSD data to examine the safety of thimerosal-containing vaccines
Managed Care Organization Sites
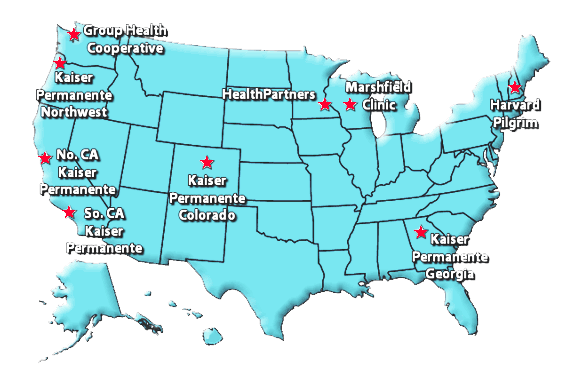
- Group Health Cooperative of Puget Sound, Seattle, WA
- Kaiser Permanente Northwest, Portland, OR
- Kaiser Permanente Medical Care Program of Northern California, Oakland, CA
- Southern California Kaiser Permanente Health Care Program, Los Angeles, CA
- HealthPartners Research Foundation, Minneapolis, MN
- Marshfield Clinic Research Foundation, Marshfield, WI
- Kaiser Permanente Colorado, Denver, CO
- Harvard Pilgrim Health Care, Boston, MA
- To conduct population-based research on immunization safety questions
- To evaluate immunization safety hypotheses that arise from medical literature, passive surveillance systems, adjustments to immunization schedules, and introduction of new vaccines
- To guide national immunization policy decisions
- To partner with healthcare providers, public health officials, and others to ensure the public has the best available information regarding the risks and benefits of immunization
Rapid Cycle Analysis (RCA) is an active surveillance system designed to detect adverse events (possible side effects) following vaccination in near real time so the public can be informed quickly of possible risks.

No comments:
Post a Comment